9 Protons 10 Neutrons 10 Electrons Total Charge
What is the net charge of an ion that has 8 protons 9 neutrons and 10 electrons. Answer 1 of 4.

Here We Ll Look At How Nonmetal Atoms Form Ions Ppt Download
View the full answer.
. -Total mass of an individual atom-Protons Neutrons. Since in this case the electrons are 10 and protons are 9 the 9 charge protons and 9- charge electrons cancel each other giving a final charge of negative. The mass number of an element that has 18 protons 18 electrons and 19 neutrons is _____.
Finding Atomic Mass Example. Start studying Protons Neutrons Electrons. What is the charge of a particle that has 9 protons and 10 electrons.
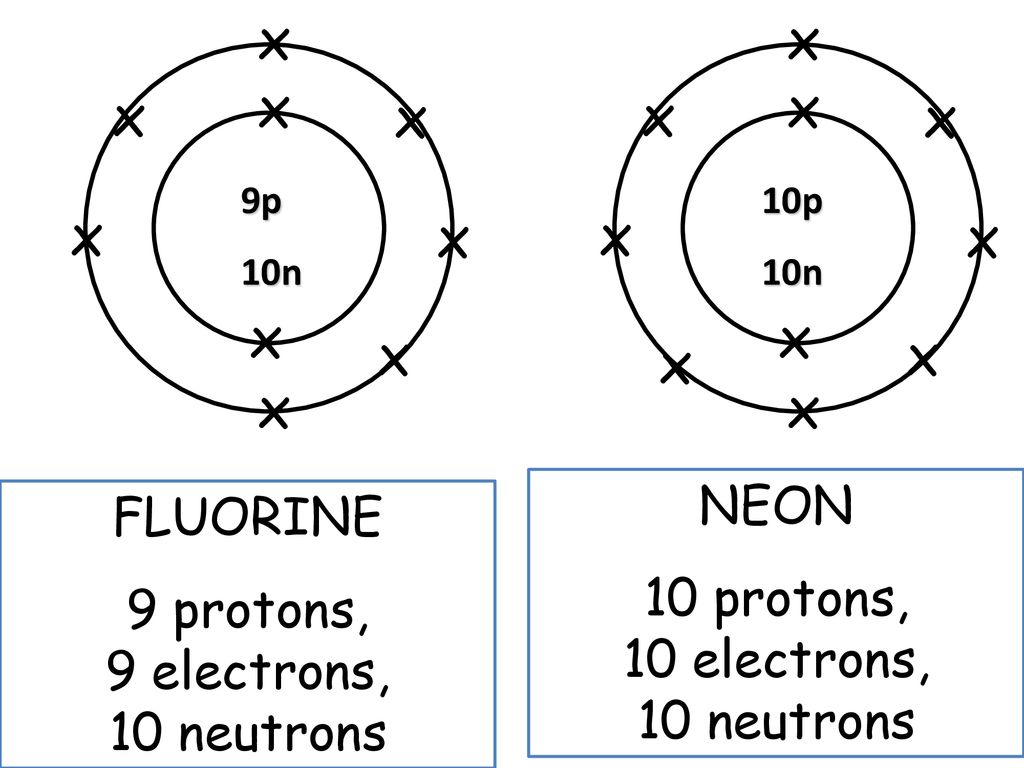
Atom of an element has 9 electrons 9 protons and 10 neutrons what is atomic no. Thus if an atom of an element has nine protons then its atomic number is also nine. Compared to the radius of a chlorine atom the radius of a chloride ion is a.
9 -10 -1. Let one charge unit be denoted. So ten protons will balance out ten electrons making the net charge 0.
Elements are defined by the number of. Learn vocabulary terms and more with flashcards games and other study tools. You need to understand what is inside those circle which you use to.
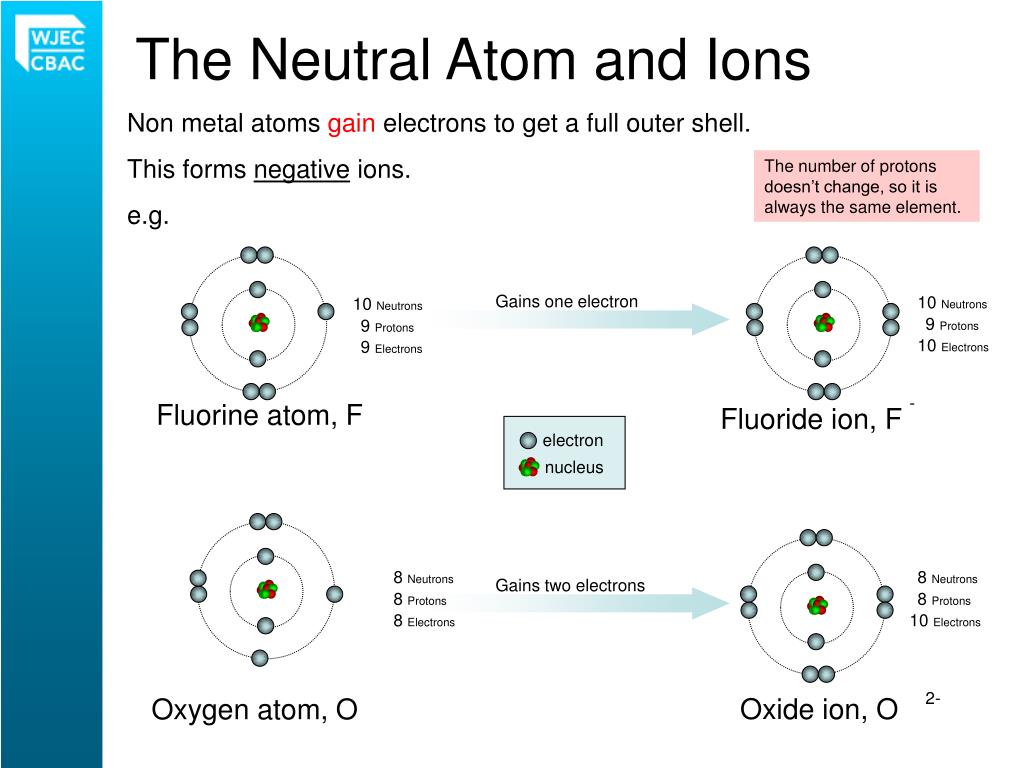
Have same amount of positive charge from protons and. If a particle has 9 protons 9 electrons and 10 neutrons it is neutral. What is the net charge of an ion that has 8 protons 9.
To be precise your question should be What isotope has 9 protons and 10 neutrons. Boron-10 199 Boron-11 801 0199010 080111 10801 amu. Any atom whose number electrons are more than the number of protons in it will have a negative charge.
The atomic number of an element that has 9 protons 9 electrons and 10 neutrons is _____. Since the atom is do troll and then well take the total number of protons plus neutrons minus the number of protons to find 34. So if we take that value and subtract the number of protons which is nine do you find the number of neutrons which is 10 in this case Then for part B will do the same thing.
Answer 1 of 2. Neutron number plus atomic number equals atomic mass number. There are only 12 Baryons in any atom in total and half of those are Neutrons.
What element has 9 protons and 10 neutrons. The total electrical charge of the nucleus is therefore Ze where e elementary charge equals to 1602 x 10-19 coulombs. In this case the Protons have an average of 2 units of charge.
What is the net charge of the ion. Protons have a positive charge while electrons have a negative charge neutrons have no charge at all. Feb 18 2018 The atomic number is 9.
This is the best answer based on feedback and ratings. Posted on April 18 2022 by. This problem has been solved.
1 9 protons 10 neutrons 10 electrons neutron having doesnot have charge it is neut View the full answer Transcribed image text. The number on the bottom is 29 so we have 29 protons and also 29 electrons. Larger because chlorine loses an electron b.
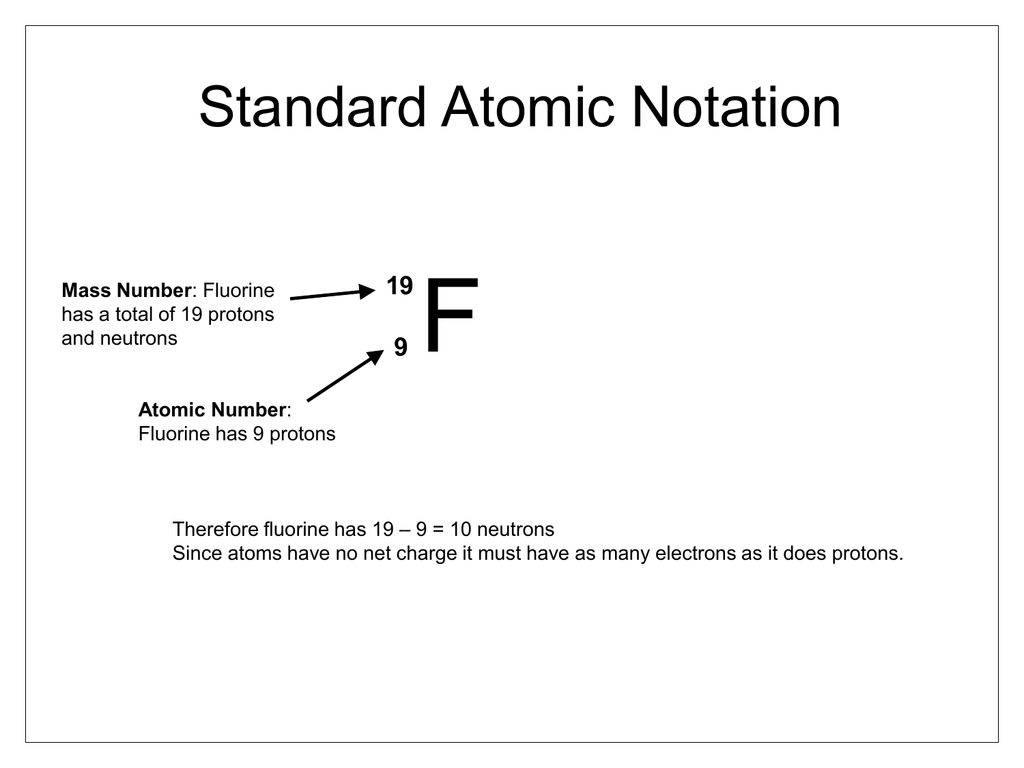
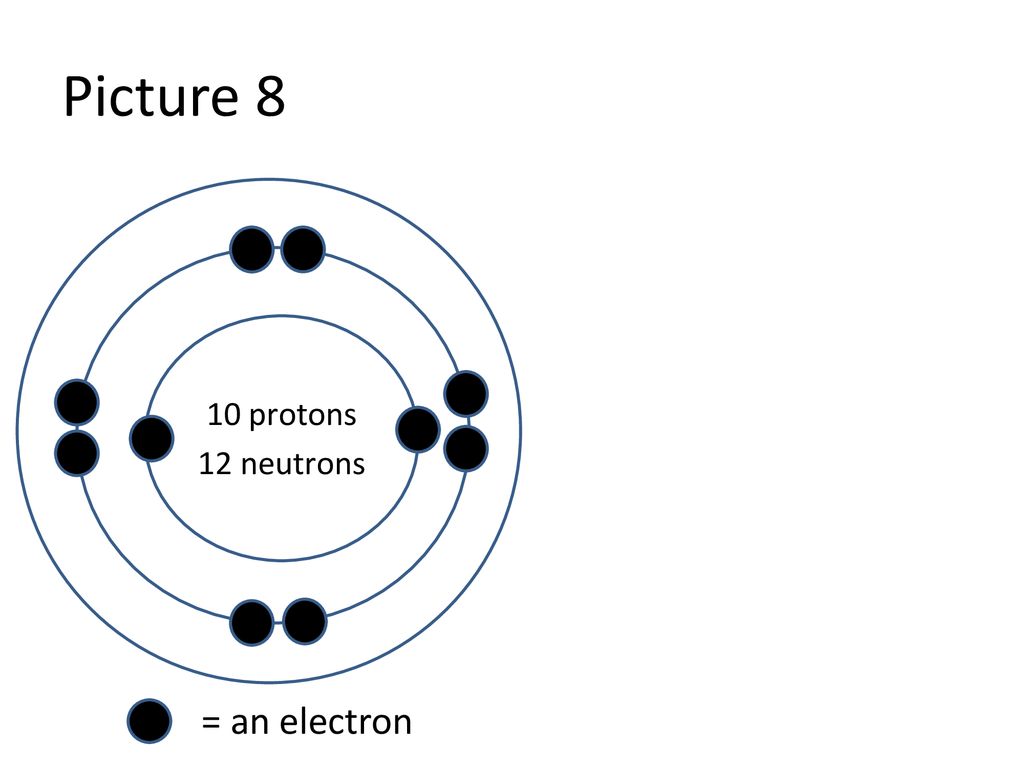
The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. The __________ of an atom is the sum of the protons and neutrons in the nucleus of that atom. Boron 10 protons neutrons electrons.
Larger because chlorine gains an electron c. Thank you for the request to answer your question Rahim. Here the number of protons is 9 hence the atomic number of X is 9.
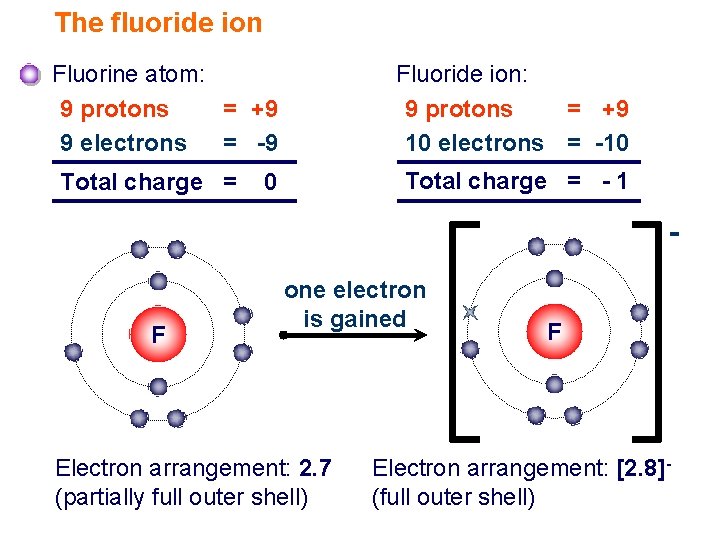
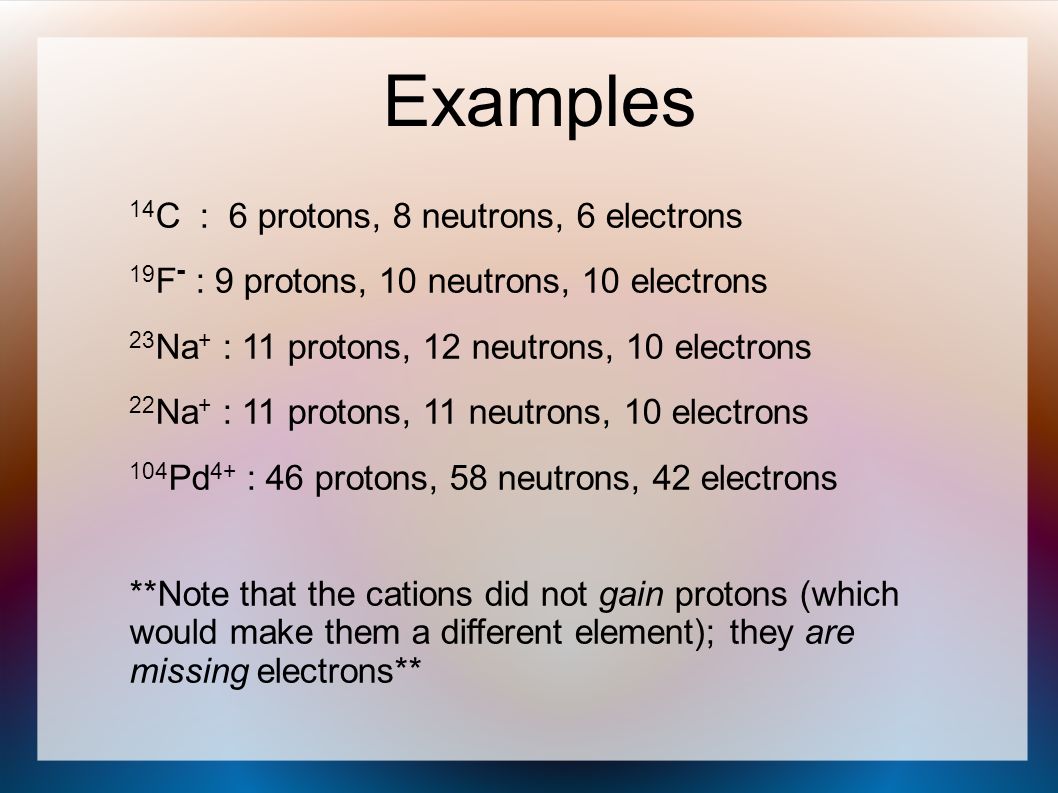
The atomic number of an element is the number of protons in one atom of that element. See the answer See the answer done loading. A flourine ion contains 9 protons 9 neutrons and 10 electrons.
There are protons and neutrons in a nucleus and there are more neutrons than protons therefore its neutral carly3110 carly3110 09242020 Chemistry High School answered An atom has 9 protons 10 neutrons and. A number of electrons are 10 means it has gained one electron from another atom hence it is an anion with 1 charge. The answer is obviously 12 but the model is naïve.
Number of neutrons does not affect the charge. Neon has 10 protons 10. A fluorine ion contains 9.
112 rows Fluorine has 9 protons 10 neutrons and 9 electrons.

Solved F 20 A Radioactive Isotope Of Fluorine Has Protons 10 Neutrons And 1 Electron Protons 10 Neutrons And 9 Electrons 9 Protons 11neutrons And 9 Electrons 10 Protons 9 Neutrons And 1 Electron 10 Protons 10 Neutrons And 10 Electrons
Ionic Bonding Elements Are The Simplest Substances There

Starter Complete The Word Wheel Write The Keywords In A Short Paragraph But They Must Be In The Order Of The Word Wheel Electrons Atomic Mass Protons Ppt Download

A Particular Ion Of Oxygen Is Composed Of 8 Protons 10 Neutrons And 9 Electrons In Homeworklib
Standard Atomic Notation Ssfa Mskahan

1 Atomic Structure And The Elements 2 What Is An Atom An Atom Is The Smallest Particle Of An Element Atoms Make Up All Matter Amu Ppt Download

Solved A Fluorine Ion Contains 9 Protons 10 Neutrons And Chegg Com
Ppt Atomic Structure Powerpoint Presentation Free Download Id 4947245
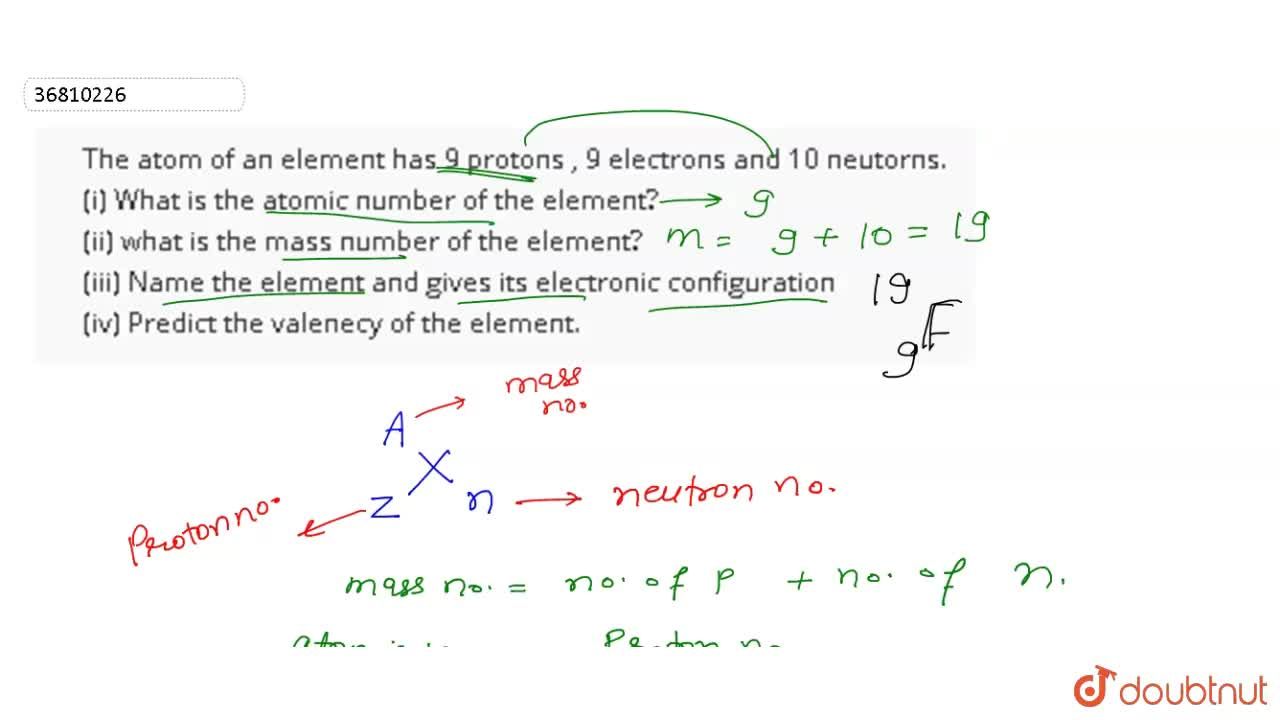
The Atom Of An Element Has 9 Protons 9 Electrons And 10 Neutorns I What Is The Atomic Number Of The Element Ii What Is The Mass Number Of The Element

Today Atomic Particles Electrons Protons Neutrons Ppt Download
Ions And Isotopes Ppt Video Online Download

Ionic Bonding Elements Elements Are The Simplest Substances There Are About 100 Different Elements Each Element Is Made Up Of Just One Particular Type Ppt Download

Year 11 Mah Matter Is Used To Describe All Materials In Our World May Be Solid Liquid Or Gases Atoms Are The Building Blocks Of Matter Very Small Ppt Download

The Atom Of An Element Has 9 Protons 9 Electrons And 10 Neutorns I What Is The Atomic Number Of The Element Ii What Is The Mass Number Of The Element

Number Of Protons Atomic Number Number Of Protons

Solved One Ion Of Axygen Consists Of 8 Protons 10 Neutrons Chegg Com
Atom Of An Element Has 9 Electrons 9 Protons And 10 Neutrons What Is Atomic No Socratic

Comments
Post a Comment